Electron Configuration Chart for All Elements in the Periodic Table. There are 118 elements in the periodic table. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. Download excel for mac free trial.
/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)
The elements of the periodic table sorted by atomic number
click on any elements name for further chemical properties, environmental data or health effects.
The latest tweets from @ricegum. Ricegum twitter.
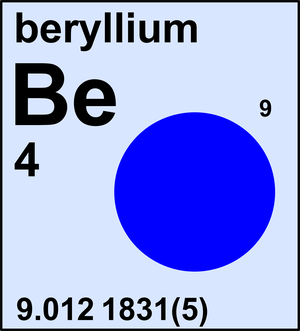
- The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The atomic mass (number at the bottom) is the amount of protons and neutrons added together. Whichever you know, you subtract from the atomic mass.
- Atomic number is number of protons whereas atomic mass is the sum of number of protons and neutrons in the nucleus. Atomic number increases regularly by one in going from one element to the next element but atomic mass does not vary regularly must of the chemical properties of elements depend upon valence electrons and electric configuration of an atom which in turn depends on the number of.
- The number of atoms or molecules (n) in a mass (m) of a pure material having atomic or molecular weight (M) is easily computed from the following equation using Avogadro's number (NA = 6.022×10 23 atoms or molecules per gram-mole): M mN n A (1) In some situations, the atomic number density (N), which is the concentration of atoms or molecules per.
- The atomic number of an element 'X' is 20. I)Determine the position of the element 'X' in the periodic table. Ii) Write the formula of the compound formed when 'X' reacts/combines with another element 'Y'(atomic number.
This list contains the 118 elements of chemistry.

| The chemical elements of the periodic chart sorted by: | Atomic number | Name chemical element | Symbol |
| - Name alphabetically | 1 | Hydrogen | H |
| - Atomic number | 2 | Helium | He |
| - Symbol | 3 | Lithium | Li |
| - Atomic Mass | 4 | Beryllium | Be |
| - Electronegativity | 5 | Boron | B |
| - Density | 6 | Carbon | C |
| - Melting point | 7 | Nitrogen | N |
| - Boiling point | 8 | Oxygen | O |
| - Vanderwaals radius | 9 | Fluorine | F |
| - Year of discovery | 10 | Neon | Ne |
| - Inventor surname | 11 | Sodium | Na |
| - Elements in earthcrust | 12 | Magnesium | Mg |
| - Elements in human body | 13 | Aluminum | Al |
| - Covalenz radius | 14 | Silicon | Si |
| - Ionization energy | 15 | Phosphorus | P |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 16 | Sulfur | S |
| 17 | Chlorine | Cl | |
| 18 | Argon | Ar | |
| 19 | Potassium | K | |
| 20 | Calcium | Ca | |
| 21 | Scandium | Sc | |
| 22 | Titanium | Ti | |
| 23 | Vanadium | V | |
| 24 | Chromium | Cr | |
| 25 | Manganese | Mn | |
| 26 | Iron | Fe | |
| 27 | Cobalt | Co | |
| 28 | Nickel | Ni | |
| 29 | Copper | Cu | |
| 30 | Zinc | Zn | |
| 31 | Gallium | Ga | |
| 32 | Germanium | Ge | |
| 33 | Arsenic | As | |
| 34 | Selenium | Se | |
| 35 | Bromine | Br | |
| 36 | Krypton | Kr | |
| 37 | Rubidium | Rb | |
| 38 | Strontium | Sr | |
| 39 | Yttrium | Y | |
| 40 | Zirconium | Zr | |
| 41 | Niobium | Nb | |
| 42 | Molybdenum | Mo | |
| 43 | Technetium | Tc | |
| 44 | Ruthenium | Ru | |
| 45 | Rhodium | Rh | |
| 46 | Palladium | Pd | |
| 47 | Silver | Ag | |
| 48 | Cadmium | Cd | |
| 49 | Indium | In | |
| 50 | Tin | Sn | |
| 51 | Antimony | Sb | |
| 52 | Tellurium | Te | |
| 53 | Iodine | I | |
| 54 | Xenon | Xe | |
| 55 | Cesium | Cs | |
| 56 | Barium | Ba | |
| 57 | Lanthanum | La | |
| 58 | Cerium | Ce | |
| 59 | Praseodymium | Pr | |
| 60 | Neodymium | Nd | |
| 61 | Promethium | Pm | |
| 62 | Samarium | Sm | |
| 63 | Europium | Eu | |
| 64 | Gadolinium | Gd | |
| 65 | Terbium | Tb | |
| 66 | Dysprosium | Dy | |
| 67 | Holmium | Ho | |
| 68 | Erbium | Er | |
| 69 | Thulium | Tm | |
| 70 | Ytterbium | Yb | |
| 71 | Lutetium | Lu | |
| 72 | Hafnium | Hf | |
| 73 | Tantalum | Ta | |
| 74 | Tungsten | W | |
| 75 | Rhenium | Re | |
| 76 | Osmium | Os | |
| 77 | Iridium | Ir | |
| 78 | Platinum | Pt | |
| 79 | Gold | Au | |
| 80 | Mercury | Hg | |
| 81 | Thallium | Tl | |
| 82 | Lead | Pb | |
| 83 | Bismuth | Bi | |
| 84 | Polonium | Po | |
| 85 | Astatine | At | |
| 86 | Radon | Rn | |
| 87 | Francium | Fr | |
| 88 | Radium | Ra | |
| 89 | Actinium | Ac | |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu | |
| 95 | Americium | Am | |
| 96 | Curium | Cm | |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es | |
| 100 | Fermium | Fm | |
| 101 | Mendelevium | Md | |
| 102 | Nobelium | No | |
| 103 | Lawrencium | Lr | |
| 104 | Rutherfordium | Rf | |
| 105 | Dubnium | Db | |
| 106 | Seaborgium | Sg | |
| 107 | Bohrium | Bh | |
| 108 | Hassium | Hs | |
| 109 | Meitnerium | Mt | |
| 110 | Darmstadtium | Ds | |
| 111 | Roentgenium | Rg | |
| 112 | Copernicium | Cn | |
| 113 | Nihonium | Nh | |
| 114 | Flerovium | Fl | |
| 115 | Moscovium | Mc | |
| 116 | Livermorium | Lv | |
| 117 | Tennessine | Ts | |
| 118 | Oganesson | Og |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Please report any accidental mistake in the above statistics on chemical elements
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved
The atomic number is the number of protons — positively charged particles — in the nucleus an atom of a chemical element. Elements are distinguished from one another by the numbers of these particles they have, and so each element has its own unique atomic number. The chemical properties of an element are determined by its number of electrons, but in a neutral atom, this is the same as the number of protons. Atoms can, however, gain or lose electrons to form negatively or positively charged ions, so the atomic number is defined as the number of protons, as this is always the same for a given element.
Atomic Number, Mass Number and Atomic Weight
It is possible to confuse these values, but they are quite distinct from one another. Atoms consist of a nucleus containing positively charged protons and electrically neutral neutrons, with electrons orbiting some distance away. Protons and neutrons are relatively heavy, and similar in weight, but electrons are very much lighter and contribute very little to the weight of an atom. The mass number of an atom is the number of protons plus the number of neutrons and is nearly equal to the weight of the atom.

The number of neutrons in an element can vary. Forms of an element with different numbers of neutrons are known as isotopes. For example, the most common form of hydrogen has one proton and no neutrons, but two other isotopes of hydrogen exist, deuterium and tritium, with one and two neutrons, respectively. Naturally occurring elements are often mixtures of different isotopes. Carbon is another example, consisting of isotopes with mass numbers 12, 13 and 14. These all have six protons, but have six, seven and eight neutrons, respectively.
Although 19th century chemists had established good approximations of the atomic weights of the known elements, the precise calculations are not always straightforward, due to the occurrence of different isotopes in varying proportions. Often, the atomic weight is determined as an average, based on the relative abundance of isotopes. Since some isotopes are unstable, changing over time into other elements, atomic weights can vary, and may be represented as a range, rather than a single value. Isotopes are usually represented with the atomic number at the bottom left of the chemical symbol, and the mass number, or approximate atomic weight, at the top right. For example carbon 13 would be shown as 6C13.
The Periodic Table
In the 1860s, the Russian chemist Dimitri Mendeleev worked on a table of the elements known at that time, initially listing them in order of atomic weight and arranging them in rows that grouped elements with similar chemical properties together. It had been noticed previously by other chemists that the properties of the elements, when ordered by weight, tended to repeat at more or less regular intervals. For example, lithium, sodium and potassium are all reactive metals that combine with non-metals in similar ways, while helium, neon and argon are all completely unreactive gases. For this reason, Mendeleev’s list became known as the periodic table.
Mendeleev’s first draft worked well, but there were a few inconsistencies. For example, listed in order of weight, iodine came before tellurium. The problem was that this grouped iodine with oxygen, sulfur and selenium, and tellurium with fluorine, chlorine and bromine. According to their chemical properties, the reverse should have been the case, so before publishing his table in 1869, Mendeleev simply swapped these elements round. It was not until the early 20th century, however, that the reason for these inconsistencies was revealed.
Atomic Number Of Beta Particle
In 1913, the physicist H.G.J. Moseley established a relationship between the wavelengths of X-rays produced by different elements and their sequence in the periodic table. As the structure of the atom was revealed by other experiments around this time, it became clear that this relationship was dependent on the number of protons in an element’s nucleus, in other words, its atomic number. The periodic table could then be ordered by this number, putting the observed chemical properties of the elements on a sound theoretical basis. The occasional inconsistencies in the original table were due to the fact that variations in the number of neutrons could sometimes result in an element having a higher atomic weight than another element with a higher atomic number.
List Of Elements By Atomic Number
The modern periodic table shows the elements in boxes arranged into rows and columns, with atomic number ascending along each row. Each column groups together elements with similar chemical properties. The columns are determined by the number and arrangement of electrons in the atoms, which in turn is determined by the number of protons. Each box normally contains the chemical symbol for the element, with the atomic number above.
